Background:
In recent years, immunosuppressive therapy (IST) in combination with thrombopoietin receptor agonist (TPO-RA) with or without other hematopoietic interventions has emerged as the first-line treatment option for patients with severe aplastic anemia (SAA) who are not eligible for hematopoietic stem cell transplantation. Hetrombopag, an innovative orally administered, non-peptide, small-molecule TPO-RA, has shown impressive efficacy and safety as a monotherapy for managing patients with relapsed or refractory SAA. Despite these promising findings, there is a lack of comprehensive real-world evidence on the benefit of hetrombopag in combination with IST-based therapy for patients with SAA. This study aims to fill this knowledge gap by evaluating the effectiveness and safety of hetrombopag-containing therapy for patients with SAA in a real-world setting.
Methods:
In this multicenter, real-world study, the clinical records of SAA patients who underwent hetrombopag-containing therapy were retrospectively reviewed in six hematological medical centers across China from August 2021 to November 2022. The therapeutic regimen encompassed cyclosporine (CsA) and hetrombopag, with the optional addition of anti-thymocyte globulin (ATG) and/or stanozolol according to the patients' condition. Hetrombopag was initially administered at a daily dose of either 5 or 7.5 mg, which could be escalated to a maximum of 15 mg daily. CsA was introduced at a preliminary dosage of 3-5 mg/kg/day, later tailored to sustain a trough concentration between 150 and 250 μg/L. ATG was given at doses of 2.5-3.5 mg/kg/day for rabbit ATG, or 20-30 mg/kg/day for pig ATG, over five days. Stanozolol was administered at 2-6 mg/day. Hematologic response (HR) was characterized based on evaluations of platelet (PLT), hemoglobin (HGB), and neutrophil (ANC) counts. PLT response was defined as an elevation to 20×10 9/L above the baseline or maintenance ≥20×10 9/L without transfusion for ≥8 weeks. HGB response was defined as an increase by 1.5 g/dL above the baseline, or for those receiving transfusions, an absolute decrease of ≥ 4 units of transfusions over an unbroken 8-week period, relative to that in the 8 weeks preceding treatment. ANC response was classified as a doubling from the baseline, or an increment in ≥ 0.5×10 9/L. Fulfillment of all three response criteria was classified as a complete response (CR), whereas meeting at least one of the three was considered an HR.
Results:
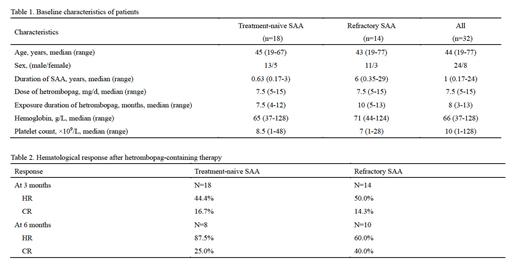
Our study comprised thirty-two patients diagnosed with SAA, of which 18 were treatment-naive, and 14 were refractory to previous IST. These patients were administered a median dose of hetrombopag of 7.5 mg (range: 5-15 mg) daily for a median duration of 8 months (range: 3-13 months). In treatment-naive SAA patients, the HR and CR rates after three months of hetrombopag-containing therapy were 44.4% and 16.7%, respectively. These rates increased to 87.5% for HR and 25.0% for CR after six months. Meanwhile, refractory SAA patients exhibited HR and CR rates of 50.0% and 14.3% respectively, after three months of therapy, and these rates improved to 60.0% and 40.0% at the six-month mark. Significantly, no patients developed hepatic or renal toxicity of grade 3 or higher. Additionally, no cases of clonal evolution or serious adverse events were documented throughout the treatment period.
Conclusions:
The hetrombopag-containing therapy demonstrated remarkable effectiveness in both treatment-naïve and refractory SAA patients in the real-world setting, with manageable safety profile. Most notably, the response rates and depth of remission in SAA patients significantly improved over time, signifying the ongoing therapeutic advantage of extended hetrombopag-containing therapy.
Disclosures
Chang:Incyte Corporation: Current Employment.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal